Tested and certified smart medical devices for state-of-the-art healthcare
Medical devices using IoT wireless technology are revolutionizing healthcare. Smart devices such as wearables now link patients to doctors, transmitting critical medical data in real time from hospital beds and private homes as well as mobile equipment used at emergency sites and in transport vehicles. Wireless devices implanted in the body permit patients to be continuously monitored to alert health care providers of changes that may require necessary action. Smart personal medical devices allow patients unprecedented mobility while providing clinicians real-time data to ensure patients the highest level of care. Smart medical devices are held to strict regulations and must be tested and certified according to established requirements such as FCC in the US market and RED compliance for the EU market.
We provide comprehensive testing services to meet various regulatory requirements designed to certify the quality, compliance, reliability, and safety of your smart medical devices. Our experts conduct interoperability and performance testing to ensure the electromagnetic compatibility, cyber security, and functional safety of smart devices to be used in the modern healthcare industry. We test the compatibility of your products with multiple short- and long-range connectivity technologies used to connect essential equipment and transfer critical data. Our global network of experts is on the ground around the world to provide you the localized support and testing expertise you need to successfully launch your smart medical device in the markets of your choice. We are a designated Notified Body in the European Union for various directives including Radio Equipment Directive (RED) along Medical Device Regulations (MDR), FCC TCB for the US market. Our experts and global lab network can offer comprehensive regulatory services for various medical devices.
Certified smart medical devices for fast access to any market
Our services have been developed to ensure the compliance, performance, and safety of your smart IoT medical devices. We test and certify your products according to recognized interoperability, security, and safety standards. We conduct accredited services for diverse international approvals according to established network protocols. We help you understand the technical requirements relevant to your smart medical devices and provide you reliable expert support throughout your certification process. We are able to serve you at anytime, anywhere in the world from a single source with our Market Access Services.
Smart medical device testing and certification
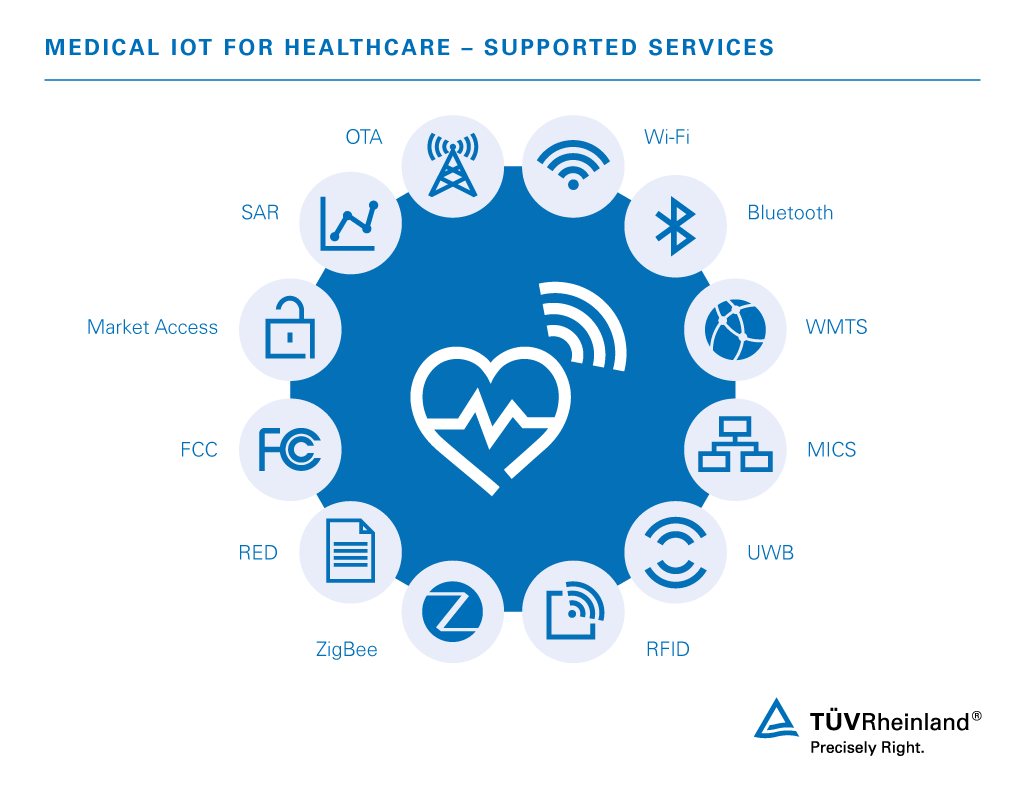
We conduct comprehensive testing and certification for wireless smart IoT medical devices. We provide the expert interoperability, security, safety, and performance assessments you need to attain type and network operator approvals. Our experts are well-versed in the rules and regulations governing country-specific as well as internationally recognized type approvals and certification processes to ensure your successful entry to the markets of your choice.
Our services for smart medical devices include:
- OTA (Over the Air)
- SAR (Specific Absorption Rate)
- Electromagnetic Compatibility (EMC)
- Cyber security testing and certification
- Performance testing and certification
- Medical auditing
- Product safety testing and certification
- Green services
- Market Access Services
We are accredited to provide testing services for connectivity technologies including but not limited to Zigbee®, Thread®, Wi-Fi®, Sigfox®, Bluetooth®, OCF, Wi-SUN®, and OpenADR.
Wireless technology programs used to connect essential IoT devices and transfer critical data include:
- Wi-Fi – 802.11b/g/n monitors patient vital signs, blood pressure, electrocardiogram etc. on either a hospital or home network
- Bluetooth – Retrieves patient data on handheld bedside devices and integrates key medical equipment in hospital rooms
- WMTS (Wireless Medical Telemetry Service) – Transfers data from body sensors with remote monitoring systems, operating in 600 to 1432 MHz frequency bands
- MICS (Medical Implant Communication System) – Low-power, short-range, high-data-range communication supporting diagnostic or therapeutic functions associated with medical implant devices and nearby controllers, operating at 401 to 406 MHz
- UWB (Ultra-Wide Band) – Uses low-power consumption to achieve high bandwidth connections for medical telemetry and imaging
- RFID (Radio Frequency Identification) – RFID tags are used in hospitals to continuously track equipment, patients, and doctors, and to properly monitor hospital supply stocks
- Zigbee – Monitors chronic disease, personal wellness, and personal fitness with low-power connectivity of a large number of devices into a single network as well as the development of the ZigBee Health Care for point-of-care medical device communication
Our comprehensive security assessment services provide a high level of assurance. We conduct hazard analyses to identify vulnerable design intersections in the device architecture. Our specialists evaluate components, data flow, and other architectural documents for potential weaknesses. We analyze source codes and conduct penetration as well as dynamic testing. We look forward to serving you.
Your trusted partner for smart medical devices services
Our services are designed to provide you the expertise you need when testing your smart IoT medical devices for approvals and certifications needed to access diverse global markets. We hold international accreditations recognized around the world, and are uniquely positioned to support you in any regulatory framework. Our experts provide comprehensive services tailored to your needs from a single source. We are at your side to guide you through every process to ensure your smart medical devices are reliable, compliant and safe.
Learn more about how you can benefit from our services!

Our experts are happy to assist you with any further questions, specific needs and requests.